
larutan 25 ml HCN 0,2 M dereaksikan dengan 25 ml NaOH 0,2 M. sesuai dengan reaksi: HCN (aq) + NaOH - Brainly.co.id

How to Write the Net Ionic Equation for HCN + NaOH = NaCN + H2O - YouTube

Solved 9 (16 points). Explain the following: HCN NaOH | Chegg.com

Untuk membentuk larutan buffer dengan pH = 5, maka berapa larutan NaOH 0, 1 M yang harus di - Brainly.co.id

Rangkuman Materi Contoh Soal Larutan Penyangga / Buffer & Pembahasan Kelas 11

Solved Write a net ionic equation for the neutralization | Chegg.com

How to Write the Net Ionic Equation for HCN + NaOH = NaCN + H2O - YouTube
Natrium hidroksida (NaOH) dan asam sianida (HCN de…

- Perhatikan reaksi netralisasi berikut!HCN(aq) + NaOH(aq) → NaCN(aq) + H20(0)Jika perubahan - Brainly.co.id
Berapakah pH jika 50,0 mL HCN 0,100 M dicampur dengan 50,0 mL NaOH 0,100 M (Ka=1,00•10^(-6))? - Quora
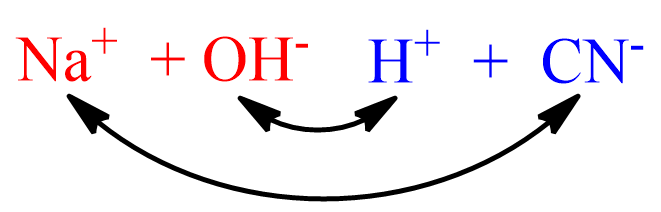
What is the net ionic equation for the rea… | Clutch Prep

Rangkuman Materi Contoh Soal Larutan Penyangga / Buffer & Pembahasan Kelas 11
Natrium hidroksida (NaOH) dan asam sianida (HCN de…

- Perhatikan reaksi netralisasi berikut! HCN(aq) + NaOH(aq) → NaCN(aq) + H200Jika perubahan entalpi netralisasi standar-12 kJ
Find the pH of a solution after 20 ml of 0.2 M NaOH is added to 80 ml of 0.15 M HCN. (Kb of CN^- = 5 × 10^-5). (log 2 = 0.3) - Sarthaks eConnect | Largest Online Education Community

Solved 9 (16 points). Explain the following: HCN NaOH | Chegg.com

PPT - Topic 4 Chemical Reactions PowerPoint Presentation, free download - ID:2144889

Net Ionic Equations HCl(aq) + NaOH(aq) NaCl(aq) + H 2 O(l) What really happens: H + (aq) + OH - (aq) H 2 O(l) Sodium ion and chloride ion are “spectator. - ppt download

Perhatikan reaksi netralisasi berikut! HCN(aq) + NaOH(aq)…

- 200ml larutan HCN 0,2M bereaksi dengan NaOH 0,1 M menghasilkan pH=5. Berapa volume NaOH jika Ka HCN = 10-5? a. 100ml b. 150ml c.

29.The enthalpy of neutralization of HCN by NaOH is -12.13k3mol. The en of HCN will be ionisation A. 4.519 kJ B. 45.10 kj C. 451.9 kJ D. 45.19 kJ

Exhaust Gas Pengobatan Memurnikan Peralatan Scrubber Basah Untuk Menghilangkan Hcl Hf Nh3 H2so4 Cro3 Hcn Naoh H2s Hcho - Buy Air Scrubber Basah Unit Untuk Limbah Pengolahan Air,Cina Industri Air Scrubber Untuk

larutan NaOH 0,1 M ditambahkan ke dalam 200 mL larutan HCN 0,05 M (ka HCN = 1×10^-7) sehingga pH - Brainly.co.id
![Solved] 17. A 1.00 L buffer solution contains 0.150 mol of HCN and 0.150 mol of KCN. A small amount of NaOH (0.03 moles) is added to the buffer solu… | Course Hero](https://i0.wp.com/www.coursehero.com/qa/attachment/3081485/ )
Solved] 17. A 1.00 L buffer solution contains 0.150 mol of HCN and 0.150 mol of KCN. A small amount of NaOH (0.03 moles) is added to the buffer solu… | Course Hero
Natrium hidroksida (NaOH) dan asam sianida (HCN de…

Hitunglah pH larutan dari: a. Larutan NaCN 0,1 M (Ka HCN = 4 x 10-6) b. CH3COONH4 0,1 M (KA CH3COONH4 = 1 x 10-5 dan Kb NH3 = 1 x 10-5) - Primalangga

Hitunglah pH larutan dari: a. Larutan NaCN 0,1 M (Ka HCN = 4 x 10-6) - Mas Dayat

Acid Base Titrations AP Chemistry Chapter 15. Titration Titrations are used to determine the amount of acid or base in a solution Titrant: the solution. - ppt download

DOC) Kerjakan Soal-soal berikut ini | Muti’ah Intan - Academia.edu

Solved Question 1 3.7 pts Write a net ionic equation for the | Chegg.com

DIT VINT 12. 20ml of OJM HCN is titrated with 0.1M NaOH. The following curve

PPT - Titration Curves PowerPoint Presentation, free download - ID:5949370

conductometric titration curve of a equimolar mixture of a HCl and HCN with NaOH(aq) is : - YouTube

A buffer solution is prepare… | See how to solve it at QANDA

Complete 32,000 ton/yr Sodium Cyanide (NaCN) Plant with Super …

The enthalpy of neutralisation of HCl by NaOH IS -55.9 kJ and that of HCN by NaOH is -12.1 kJ mol^(-1). The enthalpy of ionisation of HCN is
Soal Ulangan Buffer | PDF
what happens when acetaldehyde reacts with 1 semicarbazide 2 hcn 3 i2 naoh 4 nahso3 5 dil naoh 6 phenyl hydrazine - Chemistry - TopperLearning.com | yuf8hf66

60+ Soal Larutan Penyangga Pilihan Ganda dan Jawaban

Di bawah ini terdapat beberapa larutan. a. 25 mL NaOH 0,1 M b. 25 mL HCN 0,2 M - Mas Dayat

Sebanyak 200 mL larutan HCN 0,5 M direaksikan dengan 50 mL larutan NaOH 0,2 M menurut reaksi: HCN + NaOH –> NaCN + H2O. Jika Ka

The heat of neutralization of HCl by NaOH is -55.9KJ/mol. If the heat of neutralization of HCN by NaOH is -12.1kJ/mol. The energy of dissociation of HCN is A) -43.8 kJ B)

Pembahasan Soal Kimia Nomor 42 SBMPTN 2018 Semua Kode Soal - Larutan Bufer dan Hidrolisis Garam - Urip dot Info

Answered: The preparations of two aqueous… | bartleby

gyuug Storyboard by 86d42ec8

Why do we need three equations to find the pH of NaCN, given Ka(HCN)? - Chemistry Stack Exchange

Unduh contoh soal buffer kimia kelas XI SMA PDF - DokDrive

HCN ADDITION HCN can be synthesized using NaCN and an acid (HCI, H2SO4, etc.) NaCN, HC… - HomeworkLib
Soal Kimia Larutan Kumpul DW | PDF

NaOH + HCN = H2O + NaCN | Balanced Chemical Equation

Solved NaCN(s) + H20(g) HCN(g) + NaOH(aq) 7) What can be | Chegg.com

Hcn Is A Weak Acid And So The Salt

50 ml larutan naoh 0,2 M dicampur dengan 200 ml larutan HCN 0,05 M.hitung ph larutan.ka HCN 2.10 - Brainly.co.id
![PDF] Adsorption of HCN from Pyrolysis of Tobacco Leaves with Na2CO3, NaHCO3, and Proline | Semantic Scholar](https://i3.wp.com/d3i71xaburhd42.cloudfront.net/5680b5bc5b01d1a33ba425a31a659b4281615501/4-Figure1-1.png )
PDF] Adsorption of HCN from Pyrolysis of Tobacco Leaves with Na2CO3, NaHCO3, and Proline | Semantic Scholar

SOLVED:Chem 110/111- Tutorial = Take home Questions Write the net ionic equation for the following reaction: HN03 KZc03 NaOH HF + HCI NH; > HCN NaOH > It takes 83 mL ofa

Soal larutan penyangga - PDFCOFFEE.COM

Heat of neutralisation between HCI and NaOH is 13.7kcal and between HCN and NaOH is 3kcal at 45^(@)C. Calculate the heat of ionisation of HCN

Bab7. asam dan basa

The heat of neutralization of HCN by NaOH is 13.3 KJ/mole, the energy of dissociation of HCN is - (A) 43.8 KJ (B) - 43.8 KJ (C) - 68 KJ (D) 68
The heat of neutralisation of HCl by NaOH is -55.9 KJ/mol .If heat of neutralisation of HCN by NaOH is -12.1 KJ/mol .Find the energy of dissociation of HCN ??

Hitungah pH larutan yang terbentuk dari campuran berikut: a. 100 mL larutan HCN 0,1 M + 50 mL larutan NaCN 0,2 M; Ka HCN = 4 x 10-5 b. 50 mL larutan
Natrium hidroksida (NaOH) dan asam sianida (HCN de…

acids: 0.5 mol of NaOH is added to 1.0 L of a 0.3 M HCN solution…. - HomeworkLib

Unit III - Acid/Base - Chapter ppt download

Uživatel ON—SBMPTNFESS ✨ na Twitteru: „kimia! itu kayaknya jdi 10ml H2O. caranya gimana ya ini? pake rumus m1v1=m2v2 nya buat yg no 95nya gimanaa? makasii yg mau bantuuu😥💖 huhu… https://t.co/fs0zESQ9Or “

nucleophilic-addition-mechanism-carbonyl-cpds

Laporan praktik kerja industri

Tutorial Menjawab Soal UN Kimia SKL Larutan Penyangga - Your Chemistry A+

What is the pH when “100.0 mL” of “0.100 M HCN” is mixed with “50.0 mL” of “0.100 M NaOH”…? | Socratic
Untitled

Rangkuman Materi Contoh Soal Larutan Penyangga / Buffer & Pembahasan Kelas 11

Buffer - Latihan Soal - ? ‚ Untuk membuat larutan penyangga (buffer) dengan pH sama dengan 6, ke dalam 100 cm larutan asam asetat 0,1 M harus di-tambahkan natrium asetat padat

More Acid-Base Reactions and Equilibria

8:21 0 KB/S LTE21|| CH, CH-CH=CH,Br>CH, CH… - Organic Chemistry

OneClass: A 10.0 mL sample of 0.200 M hydrocyanic acid (HCN) is titratedwith 0.0998 M NaOH. What is t…

larutan penyangga ,bufer ,SBMPTN 2015 SAINTEK no 42 - YouTube

ファイル:Titration-HCN-NaOH.jpg - Wikipedia

Hitunglah pH larutan dari: a. Larutan NaCN 0,1 M (Ka HCN = 4 x 10-6) - Mas Dayat
Pasangan Yang Dapat Membentuk Larutan Penyangga Adalah – Mudah

Answered: Write a net ionic equation for the… | bartleby
Answer on Question#38992-Chemistry-Organic Chemistry Question Answer
SAUNG KIMIA: Kumpulan Soal Latihan dan Pembahasan Kimia Hidrolisis Garam
![Asam Kuat - [DOCX Document]](https://i1.wp.com/cdn.vdocuments.pub/img/1200x630/reader020/image/20190725/55cf880a55034664618cbec7.png?t=1632037098)
Asam Kuat - [DOCX Document]

Acid-Base Analysis - GRE Subject Test: Chemistry
conductometric titration curve of a equimolar mixture of a HCl and | Filo

Uživatel ON—SBMPTNFESS ✨ na Twitteru: „kimia! itu kayaknya jdi 10ml H2O. caranya gimana ya ini? pake rumus m1v1=m2v2 nya buat yg no 95nya gimanaa? makasii yg mau bantuuu😥💖 huhu… https://t.co/fs0zESQ9Or “

Pengertian, Ciri-Ciri dan Contoh Larutan Elektrolit dan Non Elektrolit Terlengkap | Pelajaran Sekolah Online

Chem exam on Wednesday | CHEM 1056H - Honors General Chem for Majors - Docsity

Sebanyak 8 gram kristal naoh di larutkan dalam 1 liter larutan hcn 0,3 m.bila ka= 2 x10-³dan ar na=23,o=16,h=1 , maka besarnya ph

OneClass: For a solution equimolar in HCN and NaCN, which statement isfalse?A.Addition of NaOH will i…

Pengaruh Perlakuan Garam-Garam Kalsium (Cacl2, Ca(Oh)2, Cao, Caco3) Terhadap Penurunan Kadar Hcn Tahu Dan Tepung Termodifikasi Koro Pedang (Canavalia Ensiformis).

Larutan Buffer

Pengertian Larutan Garam, Sifat, Ciri, Jenis dan Contohnya

Which pair of compounds will form a buffer in aqueous soluti… | Clutch Prep

Tutorial Menjawab Soal Tentang Jenis dan Cara Membuat Larutan Penyangga - Your Chemistry A+

Exhaust Gas Treatment Purifying Equipment Wet Scrubber To Remove Hcl\hf\nh3\h2so4\cro3\hcn\naoh\h2s\hcho - Buy Wet Scrubber,Remove Hcl\hf\nh3\h2so4\cro3\hcn\naoh\h2s\hcho,Corrosive Gases Product on Alibaba.com
Latihan Buffer

Pasangan Yang Dapat Membentuk Larutan Penyangga Adalah – Mudah

Pembahasan Soal Kimia Nomor 42 SBMPTN 2018 Semua Kode Soal - Larutan Bufer dan Hidrolisis Garam - Urip dot Info
![Solved] Data Table solution PH solution PH NH4CI 5.21 CH3COOH (HAc) 2.87 NaHCO3 8.16 HCN 5.11 KNO3 7.00 NaOH 12.92 NH3 11.11 NaCN 10.99 H2SO4 1.00 N… | Course Hero](https://i3.wp.com/www.coursehero.com/qa/attachment/13633180/ )
Solved] Data Table solution PH solution PH NH4CI 5.21 CH3COOH (HAc) 2.87 NaHCO3 8.16 HCN 5.11 KNO3 7.00 NaOH 12.92 NH3 11.11 NaCN 10.99 H2SO4 1.00 N… | Course Hero





