
berdasarkan reaksi:Fe2O3+3CO-2Fe+3CO2 yang bertindak sebagai reduktor adalah - Brainly.co.id

Fe2O3 + 3CO → 2Fe + 3CO2 Tolong dibuatkan diagram redoks nya - Brainly.co.id

Fe2O3 + 3CO → 2Fe + 3CO2 ∆H° = −28 kJ 3Fe2O3 + CO → Fe3O4 + CO2 ∆H° = −59 kJ Fe3O4 + CO → 3FeO + - Brainly.co.id

Fe2O3+3CO –2Fe+3CO2 redox reaction,in Tamil, Oxidation number#chemistrybyvalli - YouTube

Tuliskan biloks Fe dan C pada senyawa berikut Fe2o3 + 3Co menjadi 2Fe + 3CO2 - Brainly.co.id

Perhatikan reaksi redoks berikut!Fe2O3 + 3CO → 2Fe + 3CO2A. Tuliskan biloks Fe dan C pada senyawa - Brainly.co.id

Solved Which element is oxidized in this reaction? Fe2O3 + | Chegg.com

Fe2O3+3CO–>2Fe+3CO2 hasil reduksi dan oksidasi - Brainly.co.id

Tentukan perubahan bilangan oksidasi, oksidator dan reduktor pada reaksi redoks: Fe2O3 + 3CO => - Brainly.co.id

Name the substance oxidised and the substance reduced, and also identify the oxidising agent and reducing agents in the following reaction (a) 3MnO2 + 4Al → 3Mn + 2Al2O3 (b) Fe2O3 + 3CO →| Flash Education

Fe2O3+3CO—>2Fe+3CO2 carilah a. Oksidator b. Reduktor - Brainly.co.id

tentukan reduktor, oksidator, hasil reduksi, dan hasil oksidasi ! a). Fe2O3 (s) + 3 CO(g) → - Brainly.co.id
Justify that the following reactions are redox reactions: Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g)
Besi diproduksi oleh reduksi besi (III) oksida menggunakan karbon monoksida. Fe2O3(s) + 3CO(g) 2Fe(s) + 3CO2(g). Berapa banyak Fe yang dihasilkan dari 1 kg Fe2O3? - Quora
The following two reactions are known Fe2O3(g) + 3CO(g) → 2Fe(s) + 3CO2(g) ; ΔH = -26.8 kJ - Sarthaks eConnect | Largest Online Education Community
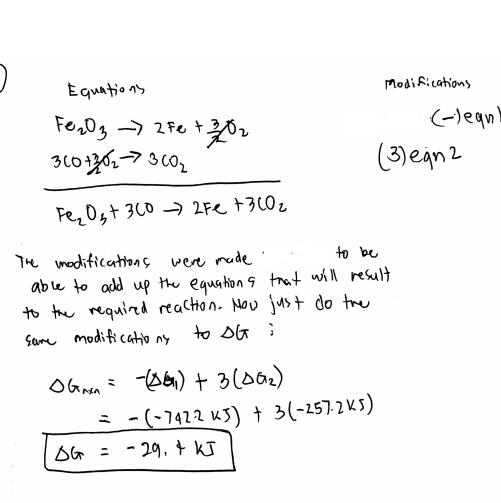
Determine ΔG° (kJ) for the following react… | Clutch Prep

Solved Given the following reactions Fe2O3 (s) + 3CO (s) → | Chegg.com

Fe²O³ + 3CO - 2Fe + 3CO² tentukan oksidator,reduktor,hasil reduksi dan hasil oksidasi - Brainly.co.id

Tentukan perubahan bilangan oksidasi, oksidator dan reduktor pada reaksi redoks: Fe2O3 + 3CO => 2Fe + 3CO2
Calculate the enthalpy change for the reaction Fe2O3 + 3CO → 2Fe + 3CO2 from the following data. 2Fe + 3/2 O2 → Fe2O3 ; - Sarthaks eConnect | Largest Online Education Community

reduction of iron (III) oxide by carbon monoxide takes place as follows Fe2O3 + 3CO = 2Fe + 3CO2 calculate the following

PENTING!! !Tentukan reduktor dan oksidator reaksi berikut: a. Mg + Cl2 MgCl2 b. Fe2O3 + 3CO 2Fe + 3CO2 c. SiCl4 + 2Mg

1 Fe2O3 (s) + 3 CO (g) → 2 Fe (s) + 3 CO2 (g) - ppt video online download

Perubahan entalpi untuk reaksi: 3C(s) + FeO2(s) → 2Fe(s) + 3CO(g) adalah - Mas Dayat

Calculations Based on Chemical Equations - ppt download

3CuCI2 + 2AI rightarrow 3Cu + 2AICI3 Fe2O3 + 3CO | Chegg.com

Stream Wryip – Fe2O3 + 3CO → 2Fe + 3CO2 by BOCTOK | Listen online for free on SoundCloud

3.9 Calculate standard enthalpy of the reaction Fe2O3 (s) + 3CO (g) - >2Fe (s) + 3CO2 (g) from the following data: AHº (Fe,0z) = -824.2 kJ mol-!, A Hº (CO) = -

Which element is oxidized in this reaction… | Clutch Prep

52 The following two reactions are known Fe2O3(s)+3CO(g)→2Fe(s)+3CO2(g);∆H=-26 8kJFeO(s)+CO(g)→Fe(s)+CO2(g);∆H=-16 5kJ The Value ∆H for the following reaction - Chemistry - Thermodynamics - 12552631 | Meritnation.com

Test You have 15 minutes to finish your test!. - ppt video online download

Solved 5.36 For the reaction Fe2O3(s) + 3CO(g) = 2Fe(s) + | Chegg.com

Fe_(2)O_(3) reacts with excess CO at a high temperature according to the equation below: Fe_(2)O_(3) + 3CO rightarrow 2Fe + 3CO_(2) If 6.5-0g of Fe_(2)O_(3) yields 3.85g of Fe, what is the

Identify the reducing agent in the following reactions: (a) 4NH3 +

Oxidation- Reduction Reaction “redox reaction” - ppt video online download

Identify the reducing agent in the following reactions (c) Fe2O3 + 3CO → 2Fe + 3CO2 - YouTube

Besi diproduksi dari reaksi antara bijih besi (Fe2…

Pembahasan Soal Olimipiade Kimia Siswa SCE Sesumatera Bagian Utara Tahun 2017 (Nomor 21-30) - Urip dot Info

Solved QUESTION 10 What is the correct word equation for the | Chegg.com
For the reaction, Fe2O3 + 3CO→ 2Fe + 3CO2, the volume of CO required to reduce one mole of Fe2O3 is:

How To Tell Which Element Is Oxidized In A Reaction

pada reaksi Fe2O3 + 3CO —-> 2 Fe + 3 Co2.spesi yang merupakan oksidator adalah…. - Brainly.co.id

Reaksi Oksidasi Reduksi - ppt download

Salah satu reaksi pada proses besi adalah sebagai berikut : fe2o3 + 3co → 2fe + 3co2 jika tersedia 16 ton bijih besi murni, maka
Tentukan oksidator, reduktor, hasil reduksi, dan h…

Solved 2. The equation Fe2O3 + 3C = 2Fe + 3CO, tells us that | Chegg.com

SOLVED:if 5.61 mol Fe2O3 and 6.87 mol of CO were reacted according to the following reaction how many moles of Fe would be produced? Fe2O3 + 3CO = 2Fe +3CO2
Solved QUESTION 30 Given the following reactions Fe2O3 (s) + | Chegg.com

Fe2o3+3co=2fe+3co2 what type of reaction

PPT - 9. Oxidation-Reduction PowerPoint Presentation, free download - ID:1003963

What Is The Balanced Equation For Fe2o3+co Yields Fe+co2

Solved Given: Fe2O3(s) + 3CO() ® 2Fe(s) + 3CO2(g); Hº = | Chegg.com

SOLVED:In the following reaction, the oxidation number of Fe changes from ____ to ____. Fe2O3 + 3CO -> 2Fe + 3CO2 * a) +2, 0 b) +3, 0 c) doesn’t change d) 0, +2 e) 0, +3

5 Thermochemistry 1 Copyright (c) The McGraw-Hill Companies, Inc. Permission required for reproduction or display. - ppt download

PPT - Topic # 9 Thermochemistry PowerPoint Presentation, free download - ID:4893646

Determine ΔG° (kJ) for the following react… | Clutch Prep

In this redox reaction, identify the element that was reduced. Fe

How To Balance Fe2o3 + Co = Fe + Co2

Solved An important reaction in a blast furnace used to make | Chegg.com

Write your Oxidation half reaction Fe2O3 + 3CO → 2Fe + 3CO2 Hello, may someone please help me - Chemistry - Chemical Reactions and Equations - 16597663 | Meritnation.com
Perhatikan reaksi redoks berikut! Fe2O3(s)+3CO(…

Fe2o3 + 3co 2fe + 3co2 tentukan oksidator reduktor hasil reduksi dan hasil oksidaesi pada masing masing reaksi
Solved For the redox reaction given below complete the | Chegg.com

How To Balance Fe2o3+co=fe+co2

REAKSI REDUKSI OKSIDASI KELAS XII SEMESTER GANJIL HULMAN

PPT - Stoichiometry PowerPoint Presentation, free download - ID:3254156
Solved 3. Which of the following reactions are redox | Chegg.com

Reduksi besi(iii) oksida dengan co menghasilkan besi menurut persamaan reaksi berikut. fe2o3(s)+ 3co(g) ⎯⎯→ 2fe(s) + 3co2(g)

Consider a reaction.Fe2O3+3CO=2Fe+3CO2. 10 g of Fe2O2 is reacted with 9 g of CO2. Find the limiting - Brainly.in
Solved 21. Consider this reaction used for the production of | Chegg.com

Analisis reaksi-reaksi berikut yang termasuk reaksi redoks dan bukan reaksi redoks! a. MnO2 + 4HCl - Mas Dayat

Percentage Yield. - ppt download

Reaksi Redoks: Reduksi dan Oksidasi - Aku Pintar

Solved 12. Given the following reactions Fe2O3 (s) 3CO (s) - | Chegg.com

Answered: Fe2O3 + 3CO → 2Fe + 3CO2 Problems… | bartleby

Redoks

tolong jawab dongg nomor satu pada gambar - Brainly.co.id

Writing Chemical Equations. - ppt download

YAYASAN PENDIDIKAN ISLAM AL-BARKAH (YAPISA) CIKALONGKULON - ppt download

- Given the following reactions 5) Fe2O3 (s) + 3CO (s) 2Fe (s)+ 3cO2 (g) AH-… - HomeworkLib

PPT - Hess’s Law PowerPoint Presentation, free download - ID:1534306

For the reaction Fe2O3 3CO ¾® 2Fe 3CO2 the volume of carbon monoxide required to reduce one mole of ferric oxide is? - PassingMarks

Solved Question 48 What is the equilibrium expression for | Chegg.com

Which substance is the oxidizing agent in … | Clutch Prep

Making only as much as we need PERCENTAGE

What is the reducing agent in Fe2O3 3co → 2fe 3co2?

1.pada reaksi Fe2O3 +3CO â 2Fe+ 3CO2,CO mengalami oksidasi karena. A. Jumlah ektronnya bertambah. - Brainly.co.id
Solved Given the following step reactions Fe2O3 (s) + 3CO | Chegg.com

Balance The Chemical Equation Fe2o3+co=fe+co2

Hematitic (Fe_(2) O_(3)) is an important ore of iron. The free metal (Fe) is obtained by reducing hematite with carbon monoxide (CO) in a blast furnace: Fe_(2)O_(3)(s) + 3CO(g) rarr 2Fe(s) +

- Tentukan reaksi oksidasi dan reduksi serta oksidator dan reduktor dari reaksi-reaksi berikut:a. 2Na+2H20 NaOH + H2 d. 3H2S + 2HNO3

Mining companies use this reaction to obta… | Clutch Prep

Solved Mining companies use this reaction to obtain iron | Chegg.com
![h_1091248257 - [DOC Document]](https://i3.wp.com/static.fdokumen.com/img/1200x630/reader018/reader/2020021819/577cc0f91a28aba71191cce9/r-1.jpg?t=1627219572)
h_1091248257 - [DOC Document]

Answered: Given the following data: Fe2O3(s) +… | bartleby

Balance Fe2O3 + CO = Fe + CO2 — Iron (III) Oxide + Carbon Monoxide) - YouTube

- The following two reactions are known : Fe2O3(s) + 3CO(g) 2Fe(s) + 3CO2(

Составить ионное уравнение Fe2O3+3CO=2Fe+3CO2 - Школьные Знания.com

ada yg bisa ? please ya bantuin :) - Brainly.co.id

Percent Yield - Solution Stoichiometry




