
How to Balance H2SO4 + NH4OH = (NH4)2SO4 + H2O (Sulfuric acid + Ammonium hydroxide) - YouTube

Larutan NH4OH dengan volume 200 mL ditambahkan ke dalam 200 mL larutan H2SO4 0,05 M - Mas Dayat

How to Write the Net Ionic Equation for NH4OH + H2SO4 = (NH4)2SO4 + H2O - YouTube

tulis rumus garamnyaa). NH4OH+H2SO4 —->. +2H2Ob). KOH+H2SO ——>. +H20_Yg twu tolong - Brainly.co.id

50 mL larutan NH4OH 0,1 M direaksikan dengan 25 mL H2SO4 0,1 M, maka derajat hidrolisis garam yang - Brainly.co.id

Type of Reaction for H2SO4 + NH4OH = (NH4)2SO4 + H2O - YouTube

Type of Reaction for H2SO4 + NH4OH = (NH4)2SO4 + H2O - YouTube

Larutan NH4OH 0,1 M yang volumenya 400 mL ditambahkan ke dalam 200 mL larutan H2SO4, - Mas Dayat

sebanyak 100 mL NH4OH 0,4 M dicampur dengan 100 mL H2SO4 0,1 M. apabila diketahui Kb NH3 = 10^-5, - Brainly.co.id
Solved Question 26 Which one of the following acid base | Chegg.com

pH larutan campuran 100 ml NH4OH 0,1 dengan 50 ml H2SO4 0,1 M adalah……(Kb NH4OH=3×10^-6) - Brainly.co.id

Larutan NH4OH dengan volume 200 mL ditambahkan ke dalam 200 mL larutan H2SO4 0,05 M sehingga diperoleh larutan penyangga dengan pH = 9 - 2 log 2. Jika diketahui Kb NH4OH = 10-5, berapa kemolaran larutan NH4OH tersebut? - Primalangga

sebanyak 100 mL NH4OH 0,4M dicampur dengan 100 mL H2SO4 0,1 M . Apabila diketatuhi Kb NH3=10 - Brainly.co.id

Berapa mL larutan H2SO4 0,5 M yang harus ditambahkan ke dalam 100 mL larutan NH4OH 0,5 M - Mas Dayat

Solved Show the complete and total ionic equations the | Chegg.com

Contoh Soal No. 4 Larutan Penyangga/ Buffer - YouTube

LARUTAN PENYANGGA. - ppt download

Contoh Soal No.6 Larutan Penyangga/Buffer - YouTube
Name the ions formed when – HCl ; HNO3 ; H2SO4 ; CH3COOH ; NaOH and NH4OH ionise in aq. soln. - Sarthaks eConnect | Largest Online Education Community

Nh4oh Hcl ~ 35+ images lot of apothecary bottle jar hcl h2so4 nh4oh, how to write the net ionic equation for hcl nh4oh, adding hcl to a cuso4 nh4oh solution

Solved I. BALANCE THESE EQUATIONS CORRECTLY: HNO3 + NH4OHN | Chegg.com

Which of the following pair has heat of neutralization equal to -57.1kJ? (A) HNO3, KOH. (B)HCl, NH4OH. (C) H2SO4, NH4OH. (D) CH3COOH, NaOH? | EduRev NEET Question

Ke dalam 100ml larutan NH4OH 0,2 M ditambahkan 100 ml larutan H2SO4 0,1M (Kb NH4OH=10'-5),maka pH - Brainly.co.id

Solved Balanced Equations NaCl + KNO3 -> NaCl + AgNO3 -> +12 | Chegg.com
Solved NH4OH (aq) (NH4)2SO4(aq) H2O 3. equation: H2SO4(aq) + | Chegg.com

PPT - Agenda 5-13-07 PowerPoint Presentation, free download - ID:2737546

Solved Section: 302 BALANCE THESE EQUATIONS CORRECTLY: HNO3 | Chegg.com

Besarnya pH campuran 100 mL larutan NH4OH 0,4M yang dicampurkan dengan 50 mL H2SO4 0,2(Kb - Brainly.co.id

Keseimbangan Elektrolit - ppt download
Jika diketahui Kb NH4OH = 1,8×10−5, tentukan harga…

Sebanyak 100 mL larutan NH4OH 0,8 M dicampurkan ke dalam 100 mL larutan H2SO4 0,8 M Kb=10^5. Tentukan pH campuran tersebut!

TULISKAN REAKSI IONISASI SENYAWA a. h2so4 b. CH3COOH c. NaCl - Brainly.co.id

Acid Definitions Lewis Acid BrnstedLowry Arrhenius acids Arrhenius

Balancing Chemical Equations - ppt download

Tolong dong bantu larutan nh4oh 0,1 m yang volumenya 400 ml ditambahkan kedalam 200 ml larutan h2so4 ternyata diperoleh larutan penyangga

Sebanyak 100 mL larutan H2SO4 dengan pH = 1 dicampurkan dengan 100 mL larutan NH4OH 0,2 M (Kb NH4OH - Brainly.co.id

- hitung besar ph dari 100ml 0,01m h2so4 2. hitung besar ph dari nh4oh 0,1 m (kb nh4oh = 10 pangkat -5) 3. hitung ph larutan dari

Solved QUESTION 4 What type of chemical reaction is the | Chegg.com
Tuliskan reaksi ionisasi dari senyawa berikut. a….
![20 mL of 0⋅1M H2 SO4 solution is added to 30 mL of 0⋅2 M NH4OH solution. The pH of the resultant mixture is : [pkb of NH4OH = 4⋅7]. - Sahay LMS](https://i0.wp.com/sahay.guru/wp-content/uploads/2020/11/ans-7.jpg)
20 mL of 0⋅1M H2 SO4 solution is added to 30 mL of 0⋅2 M NH4OH solution. The pH of the resultant mixture is : [pkb of NH4OH = 4⋅7]. - Sahay LMS

How to balance H2SO4+NH4OH=(NH4)2SO4+H2O |Chemical equation H2SO4+NH4OH =(NH4)2SO4+H2O|H2SO4+NH4OH= - YouTube

- jika 100 ml h2so4 0,05 m di campurkan dengan 100 ml nh4oh 0,1 m, tetapan hidrolisis 10^-9.ph larutan tersebut a. 5-log 6 b. 6-log

berapa mL larutan H2SO4 0,5M yang harus ditambahkan ke dalam 100mL larutan NH4OH 0,5 bila larutan - Brainly.co.id
![20 mL of 0.1M H2SO4 solution is added to 30 mL of 0.2M NH4OH solution. The pH of the resultant mixture is: [ pKb(NH4OH) = 4.7] .](https://i0.wp.com/toppr-doubts-media.s3.amazonaws.com/images/4598043/7b2dd8c3-edfc-46fc-9d1a-69effbb894f3.jpg)
20 mL of 0.1M H2SO4 solution is added to 30 mL of 0.2M NH4OH solution. The pH of the resultant mixture is: [ pKb(NH4OH) = 4.7] .

Pembuatan Phenol Dari Cumene Hidroperoksida Dengan Katalis Asam Sulfat Dengan Kapasitas 20.000 Ton/Tahun

100 ml larutan h2so4 0,1 m direaksikan dengan 100ml nh4oh 0,2 m jika kb: 10^-5 , ph larutan yang terjadi

REAKSI PEMBENTUKAN GARAM - KIMIA SMA - YouTube

Konsentrasi ion hidroksida (ohmin) yang terkandung dalam larutan nh4oh 0.1 m (konstanta basa lemah nh4oh = 1.44 x 10min5) adalah?

Solved 6 M (dil) NH4OH(aq)+ 3 M (dil) H2SO4(aq) 7. CuSO4(aq) | Chegg.com

seorang siswa ingin melakukan percobaan reaksi asam dan basa. disediakan larutan 100Ml NH4OH 0,2 M - Brainly.co.id

Chemical Equation Balancing - ppt download

Titrasi Asam Basa: Menentukan Kadar Konsentrasi Larutan Asam Basa - Aku Pintar
Anion Kation | PDF | Sustancias químicas | Química
International Journal of Renewable Energy Development

Q:- 10ml of M/10 NH4OH solution is titrated with M/10 solution of H2SO4 Calculate the pH when 5ml - Chemistry - Aldehydes Ketones and Carboxylic Acids - 12379191 | Meritnation.com
Kunci Latihan Penggaraman | PDF
Solved 1. H2SO4 + BaCl2 —> + Net lonic Equation: + 2. | Chegg.com

Obtaining of the bottom product. | Download Scientific Diagram

200 ml larutan nh4oh 0,2 m direaksikan dengan 200 ml H2 so4 0,05 m akan dihasilkan larutan dengan - Brainly.co.id

CHEMICAL SCREENING AND ANTIMICROBIAL ACTIVITY OF EXTRACTS FROM THE FLOWERS AND STEM OF HYPHAENE THEBAICA L.(ARECACEAE) | Semantic Scholar

pH LARUTAN PENYANGGA - KIMIA SMA - YouTube

Disajikan beberapa larutan berikut1. 50 mL NH4OH 0,1 M2. 50 mL H2SO4 0,05 M3. 50 mL KOH 0,05 M4. 50 mL HNO3 0,1 M5. 50 mL HCOOH 0,1
Solved 1. HNO, + NH,OH → NH.NO, + HO 2. NH4OH + H2SO4 | Chegg.com
Petunjuk Penulisan Artikel Untuk Jurnal Teknika (14 pt)
Jurnal Riset Pendidikan Kimia

Harga Ph campuran dapat diukur jika 100 ml larutan H2So4 0,2M dicampur dengan 400 larutan NH4OH - Brainly.co.id

Reagents used during decomposition methods tested c/(mol L −1 ) Na 2 CO… | Download Table

Match the neutralization reaction to the salt formed. A. NH4Cl , 2KOH + H2SO4 —>_+2 H20… - HomeworkLib

Acid- Base Neutralization Reactions - ppt download

Solved Application of Principles 1. What substance could you | Chegg.com

KIMIA KELAS 11 MENGHITUNG PH LARUTAN PENYANGGA. 1. Moza membuat suatu larutan penyangga dengan mencampur kan sebanyak 100mL NH4OH

Berapa mL volume NH4OH 0,1 M dan HCl 0,05 M masing-masing harus dicampurkan agar diperoleh 400 mL larutan penyangga dengan pH = 9 - 2 log 2? (Kb NH4OH = 10-5) PEMBAHASAN - Primalangga
kejarcita | #1 Bank Soal Sekolah
Sebanyak 100 mL H2SO4 0,1 M dicampur dengan 100 m…
![SOLVED] 16. 20 ml of 0.1 M H2SO4 is added to 30 ml of 0.2 M NH4OH then calculate pH of resultant solution. (Given that pkb of NH4OH is 4.7) 0.1 M](https://i3.wp.com/toppr-doubts-media.s3.amazonaws.com/images/3888854/e43146bf-d13c-43f9-90bf-834804496027.jpg )
SOLVED] 16. 20 ml of 0.1 M H2SO4 is added to 30 ml of 0.2 M NH4OH then calculate pH of resultant solution. (Given that pkb of NH4OH is 4.7) 0.1 M

- Sebanyak 90 mL larutan NH4OH 0,1 M (Kb = 10-5 ) dicampur dengan 15 mL larutan H2SO4 0,1 M, - Brainly.co.id
wkwkwk | PDF

What Is Formed Nh4oh

Rare-earth element concentration in the rare-earth hydroxide from… | Download Scientific Diagram

Document

fiolaa'25 🌻 maap lg males up progress 🥲🙏🏻 on Twitter: “15.https://t.co/NzQFRF07Vi (khusus matematika, kimia, fisika, ada rangkuman & lat.soal & beberapa ada video pembahasannya) *ini daftar isi web nya, sama contoh isi
![PDF] Qualitative Analysis of Coagulant Type in the Raw Rubber Material | Semantic Scholar](https://i3.wp.com/d3i71xaburhd42.cloudfront.net/e79c870636c410f875946db07c93182611700a2b/8-Table3-1.png )
PDF] Qualitative Analysis of Coagulant Type in the Raw Rubber Material | Semantic Scholar
LAPORAN PENELITIAN “Analisa Kadar Protein Pada Teripang (Holothuria argus) Terhadap Lama Perebusan” Oleh: Baterun Kunsah, S.

H2SO4 & NH4OH

Catatan tentang •KIMIA• (REAKSI - Clearnote

Solved equation Balanced Equations NaCl + KNO, —— Naci + | Chegg.com

Among the following the plot that correctly represents the conduc

Senyawa berikut yang menghasilkan jumlah ion paling banyak jika dilarutkan dalam air adalah.. a. hi b. hbr c. koh d. h2so4 e. nh4oh
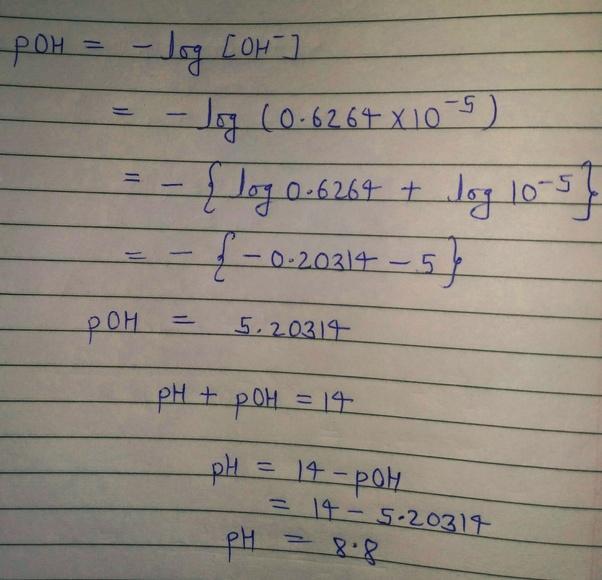
What Is The Ph Of H2so4
ANALISA KADAR PROTEIN PADA TERIPANG (Holothuria argus) TERHADAP LAMA PEREBUSAN PENDAHULUAN Protein merupakan zat gizi yang pali

Perhatikan persamaan reaksi hidrolisis berikut! …

tuliskan reaksi berikut ini:a. NH4OH + H2SO4 —> - Brainly.co.id

Penetapan Kadar Fe dalam Tawas Ferri Amonium Sulfat SMK-SMAK Bogor

20 mL of 0.1M H2SO4 solution is added to 30 mL of 0.2M NH4OH

Salt Hydrolysis Lesson ppt download

Choice 1 Pyrex Corning 500 Ml Reagent Bottles Empty Legible H2so4 Nh4oh for sale online | eBay
20 mL of 0.1 MH2SO4 solution is added to 30 mL of 0.2 M NH4OH solution. The pH of the resultant mixture is: - Sarthaks eConnect | Largest Online Education Community

PDF) KUMPULAN SOAL-SOAL UJIAN KIMIA (KIM 101) BAB 8 ASAM-BASA | dede mawadah - Academia.edu

Solved Find the balanced equation, Molecular, total Ionic | Chegg.com

PPT - Acid, Bases, and Salts PowerPoint Presentation, free download - ID:3061423



